Have you ever wondered about the tiny, invisible bits that make up everything around us? We're talking about the very foundations of matter, the little pieces that, when put together, create all the stuff you can see, touch, and even breathe. It's almost like a secret world, you know, where everything starts with something incredibly small, yet so important. These little building blocks are what give substances their particular characteristics, their very essence, in a way.
Really, when we look at the world, from the water we drink to the air we breathe, it's all made of these minute structures. They are the smallest identifiable units of a pure substance that still keep all of that substance's specific qualities. Without these tiny arrangements, nothing would quite be the same, and the way things interact would be completely different. It's pretty fascinating to think about, actually, how something so small can have such a big impact on everything.
So, what exactly are these fundamental pieces? We often hear the word, but what does it truly mean? This discussion will help you get a clearer picture of what these essential units are, how they come together, and why they matter so much in the grand scheme of things. We'll look at the simple explanation, and you'll see just how these tiny formations play a part in every single thing around us, like your chair or the very air you're breathing. It's a basic idea, but quite powerful.
Table of Contents
- What Is a Molecule at Its Core?
- The Molecule Definition and Its Connections
- How Do Atoms Form a Molecule Definition?
- Understanding the Basic Molecule Definition
- Examples of the Molecule Definition in Action
- Why Is the Molecule Definition So Important?
- What Makes a Molecule Different from an Atom?
- The Simplest Molecule Definition Explained
What Is a Molecule at Its Core?
At its very essence, a molecule is a gathering of two or more atoms that are joined together. These atoms stick to each other through what we call chemical bonds. It's almost like they're holding hands, forming a little group. This group, this little assembly, does not carry an electrical charge; it is what we refer to as electrically neutral. So, when you picture a molecule, imagine a tiny, balanced team of atoms, linked up and going about their business without any extra electrical push or pull. This is pretty much the most straightforward way to think about it, you know.
The number of atoms in one of these groups can vary quite a bit. Sometimes it's just two, sometimes many more, but the key thing is that they are connected. These connections are very specific, and they help give the molecule its unique characteristics. Without these bonds, the atoms would just be separate entities, floating around. So, the very idea of a molecule, in some respects, hinges on these attractive forces that bring and keep atoms together in a stable unit. It's really quite a fundamental concept, you see.
The Molecule Definition and Its Connections
The definition of a molecule often emphasizes the bonds that hold it together. These bonds are a kind of attractive force, a pull that keeps the atoms from drifting apart. When we say "chemical bonds," we are talking about the way electrons are shared or exchanged between atoms, creating a stable link. This connection is what transforms individual atoms into a single, cohesive chemical entity. It's not just a random collection; it's a very particular arrangement, you know, a specific kind of linking that makes it a molecule.
This idea of being "electrically neutral" is also a big part of the molecule definition. It means that the total positive charges from the atomic nuclei are balanced out by the total negative charges from the electrons. So, the whole group, as a unit, doesn't have a net positive or negative charge. This neutrality is a distinguishing feature, helping us tell molecules apart from other types of atomic groupings, like ions, which do carry an electrical charge. It's a subtle but important point, actually, when we talk about what makes a molecule, well, a molecule.
How Do Atoms Form a Molecule Definition?
Atoms get together to form molecules when they create these chemical bonds with each other. It's a bit like building with tiny, tiny blocks. Each atom has a certain number of electrons that move around its center, its nucleus. These electrons are key to forming the connections. When atoms come close enough, their electrons can interact, either by being shared between them or by one atom completely giving up an electron to another, and stuff. This sharing or giving and taking is what creates the attractive force, the bond, that holds them together as a single unit.
So, a molecule is basically two or more atoms that have decided to stick together in this specific way. They're not just near each other; they are truly linked. This linkage results in a new, larger entity that has its own set of characteristics, different from the individual atoms it's made from. It's a pretty neat trick, really, how these tiny particles combine to create something new and distinct. You know, it's how all the different substances in the world come to be.
Understanding the Basic Molecule Definition
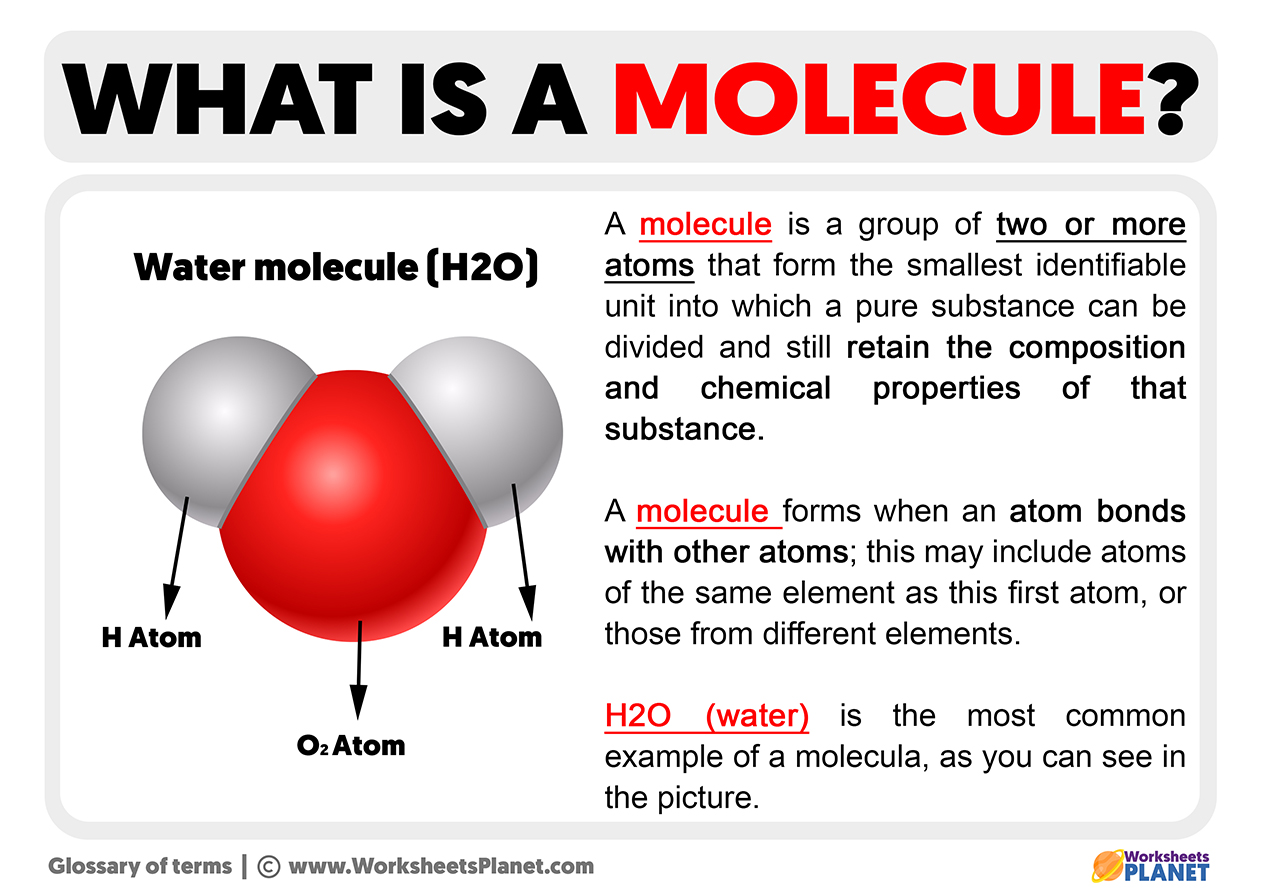
The most straightforward way to grasp the molecule definition is to think of it as the smallest piece of a substance that still behaves like that substance. Imagine you have a glass of water. If you keep dividing that water into smaller and smaller bits, you'll eventually reach a point where if you divide it any further, it won't be water anymore. That smallest piece that is still water is a water molecule. It keeps all the properties of water, like how it acts and what it's made of. This is a pretty simple way to look at it, too.
This concept means that a molecule is the fundamental unit that retains the composition and chemical properties of a pure substance. It's not just any group of atoms; it's the specific group that, when isolated, still shows all the characteristics of the larger material it came from. So, when we talk about the meaning of molecule, we're really talking about this smallest, property-retaining particle. It's quite a precise way of looking at matter, you know, at its most basic level.
Examples of the Molecule Definition in Action
To really see the molecule definition come to life, let's consider some everyday examples. Water, as we just mentioned, is a classic. A single water molecule is made of two hydrogen atoms and one oxygen atom, all bonded together. That specific combination is what makes it water. Or think about the air we breathe. It has oxygen molecules, which are two oxygen atoms linked up, and nitrogen molecules, which are two nitrogen atoms joined. These are all examples of atoms, both the same and different, forming these tiny, distinct units. It's really quite common, you know.
Sometimes, a molecule can be made up of atoms from only one type of element. For instance, a molecule of helium is just a single helium atom, but it still functions as a molecule in that context because it's the smallest unit of helium that retains its properties. Similarly, a piece of aluminum is a pure element, and its molecule, in some respects, can be considered just a single atom of aluminum. So, while often it's two or more atoms, the core idea is about the smallest unit that keeps the substance's identity. This is a bit of a nuance, actually.
Why Is the Molecule Definition So Important?
Understanding the molecule definition is incredibly important because molecules are the fundamental pieces of chemical compounds. They are the smallest part of a compound that can actually take part in a chemical reaction. When substances interact, it's often their molecules that are doing the interacting, breaking apart and forming new connections to create new substances. Without knowing what a molecule is, it would be really hard to explain how chemicals behave or how different materials are formed. It's pretty much the starting point for chemistry, you know.
Every chemical substance, whether it's a simple element or a complex compound, has its own specific type of molecule. These molecules determine everything from how hard or soft a material is, to its color, its smell, and how it reacts with other things. So, grasping this basic idea helps us make sense of the entire physical world around us. It's a very powerful concept, truly, for explaining so much about matter.
What Makes a Molecule Different from an Atom?
It's easy to get atoms and molecules mixed up, but there's a clear difference. An atom is the most basic, smallest particle of an element that still has the properties of that element. Think of it as a single, individual building block. A molecule, on the other hand, is a group of two or more of these atoms that are held together by those attractive forces we talked about, the chemical bonds. So, while an atom is a solitary unit, a molecule is a collection, a team, of atoms. This distinction is very important, you know, for understanding matter.
For example, a single oxygen atom is just that – an oxygen atom. But the oxygen we breathe is actually an oxygen molecule, which is two oxygen atoms bonded together. The properties of a single oxygen atom are quite different from the properties of an oxygen molecule. The molecule is the stable form that exists freely and exhibits the characteristics we associate with oxygen gas. So, in some respects, the molecule is the functional unit, while the atom is the individual component. It's pretty much like the difference between a single brick and a wall built from many bricks.
The Simplest Molecule Definition Explained
To put it as simply as possible, a molecule is just two or more atoms that have formed a bond with each other. These atoms can be the same kind, like two oxygen atoms making an oxygen molecule, or they can be different kinds, like hydrogen and oxygen atoms making a water molecule. The key idea is that they are connected, not just floating separately. This connection makes them act as a single, combined unit, and this unit is the smallest part of a substance that still has all the traits of that substance. It's really that simple, actually, at its core.
This definition helps us understand the fundamental makeup of everything. From the tiniest speck of dust to the largest star, it's all built from these molecular units. They are the true basic components, the very foundation upon which all matter is structured. So, when you hear "molecule," just picture a tiny, stable group of atoms, linked together, forming the essence of a particular material. That's pretty much the whole idea, you know.
This exploration has covered the fundamental idea of what a molecule is, how it's formed by atoms connecting through chemical bonds, and why these tiny, electrically neutral groups are so essential to understanding the world around us. We looked at how molecules are the smallest parts of a substance that still keep all its properties, and how different types of atoms can combine to form them. We also touched on examples like water and elements such as aluminum and helium, showing how the molecule definition applies in various situations. The discussion also clarified the distinction between an atom and a molecule and highlighted the importance of molecules as the basic units involved in chemical reactions and the composition of all matter.

/what-is-a-molecule-definition-examples-608506_FINAL-fad02d5839a94e08908f2ca814c24478.png)